The CSF surveillance program was initiated in 2006 to rapidly detect CSF virus and monitor the risk of introduction of the virus in the U.S. swine herd. The surveillance program targets five specific swine populations for testing:
- Sick pig submissions to veterinary diagnostic laboratories (VDLs)
- Slaughter swine with a high probability of CSF exposure
- Feral swine
- Swine populations (including waste feeding operations)with a high probability for CSF exposure in Florida, Texas, and Puerto Rico
- Swine highly suspicious for CSF and entered into a Foreign Animal Disease Investigation
NAHLN laboratories conduct CSF surveillance testing and NVSL’s Foreign Animal Disease Diagnostic Laboratory (FADDL) is the CSF reference and confirmatory laboratory. In FY 2013, 18 NAHLN laboratories have provided diagnostic testing for the CSF surveillance program.
The table below shows the number of animals tested for CSF by NAHLN laboratories in two target surveillance populations in FY 2012 and the first three quarters of FY 2013. Feral swine test counts are not included here and waste feeder/high probability data are not available.
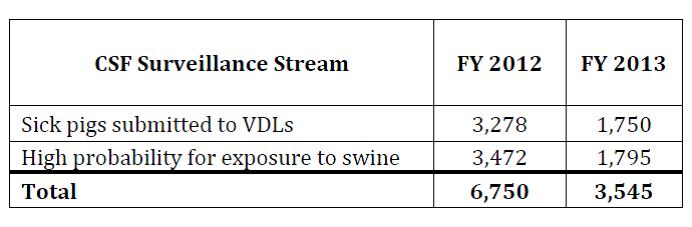
Information on feral swine serologic CSF testing can be found in the article below titled "Wildlife Services’ Surveillance and Emergency Response."
Swine Influenza A Virus Surveillance
The goals of the swine influenza A virus (IAV-S) surveillance program (formally referred to as swine influenza virus) are (1) to monitor the genetic evolution of endemic IAV-S to better understand endemic and emerging influenza virus ecology, (2) make IAV-S isolates and associated epidemiologic data available for research and analysis, and (3) select proper isolates for the development of relevant diagnostic reagents, updating diagnostic assays, and vaccine seed stock products. The program was initiated in May 2009 with a focus on monitoring the pandemic H1N1 2009 [pH1N1 (2009)] virus in swine. As the human health threat of pH1N1 (2009) declined in 2010, IAV-S surveillance efforts were re-focused on monitoring all current circulating IAV-S. Also in 2010, an anonymous submission protocol was adopted to encourage more industry participation and increase the number of samples available for monitoring IAV-S in the U.S. swine herd. IAV-S surveillance efforts are targeted towards these three swine populations:
- Case-compatible sick pig submissions to VDLs
- Swine exhibiting influenza-like illness at first points of concentration or commingling events i.e., markets, fairs
- Swine populations that are epidemiologically linked to confirmed human cases involving IAV-S
NAHLN laboratories conduct IAV-S surveillance for the above-mentioned streams. The NVSL Diagnostic Virology Laboratory in Ames, Iowa is the IAV-S confirmatory laboratory.
IAV-S testing results reported by NAHLN laboratories in FY 2012 and FY 2013
The table below shows the number of herds (accessions) tested, number of influenza-positive herds, and number of herds with virus subtyping results reported in FY 2012 and FY 2013.
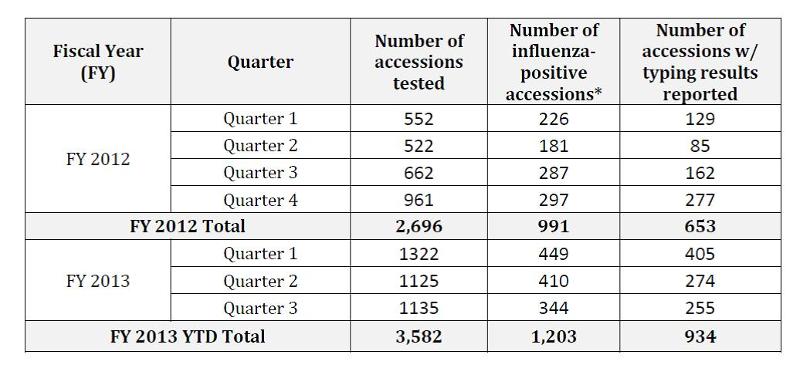
*Influenza-positive accessions are those with samples with positive matrix assay results
Pseudorabies Virus Surveillance
The PRV surveillance program was initiated in 2009 as an extension of USDA’s successful PRV eradication efforts. The program gathers surveillance data to support three specific objectives: (1) rapidly detect PRV entry and infection in U.S. commercial swine, (2) demonstrate freedom from PRV in commercial herds, and (3) monitor domestic sources of PRV.
Targeted Populations (surveillance streams)
- Investigation and diagnosis of suspicious PRV cases
- Sick pig submissions to veterinary diagnostic laboratories (VDLs)
- Herds participating in routine serology and herd profiling
- Herds classified as high probability for exposure
- Herds with reported exposure to feral swine
- Cull sow-boars at slaughter
- Market swine at slaughter
- Feral swine
PRV-approved NAHLN laboratories test serologic samples from domestic swine for four targeted PRV surveillance populations: sick pig submissions, routine serology and herd profiling, swine populations with a high probability for exposure, and swine with known feral swine exposure. NAHLN laboratories also conduct PRV testing for domestic swine samples that are submitted as part of epidemiologic trace back investigations. Twelve NAHLN laboratories currently conduct PRV surveillance sample testing in domestic swine and four NAHLN laboratories conduct PRV testing on feral swine samples (see article titled "WS Surveillance and Emergency Response"). The NVSL Diagnostic Virology Laboratory serves as the national reference laboratory and performs confirmatory testing for suspect and positive submissions for PRV.
In FY 2012, 310,569 and in the first three quarters of FY 2013, 203,342 swine samples were tested under the PRV surveillance program in the following streams: diagnostic laboratory serologic submissions, sow-boar slaughter, and market slaughter. NAHLN laboratories tested 23,928 swine for the PRV diagnostic laboratory stream in FY 2012 and 18,075 to date in FY 2013. Information on feral swine serologic submissions tested for PRV by selected NAHLN laboratories can be found in the article titled "Wildlife Services’ Surveillance and Emergency Response."
Source: NAHLN Quarterly Report